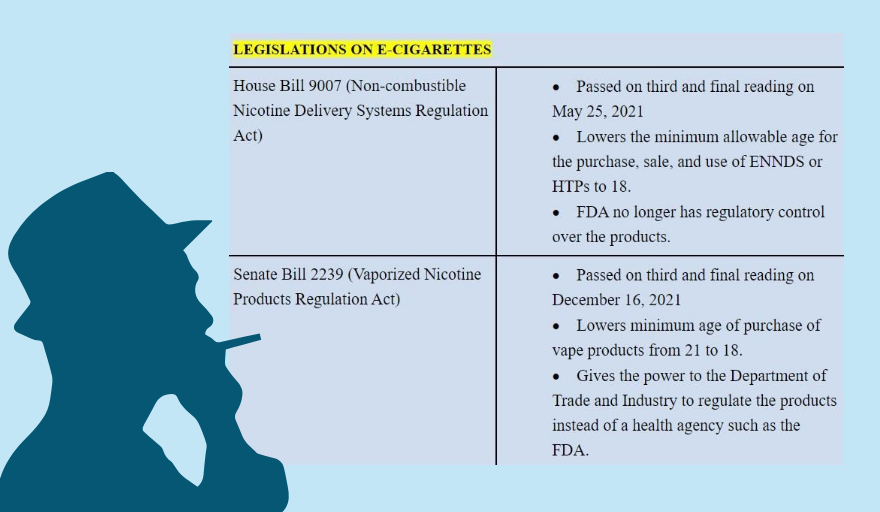
Several provisions in the proposed Vaporized Nicotine and Non-nicotine Products Regulation Act or the vape bill, ratified by Congress on Jan. 26, have been contested by medical experts and health advocates, questioning the need for a new regulation on electronic nicotine and non-nicotine devices (ENDS/ENNDS) and heated tobacco products (HTPs).
Health Secretary Francisco Duque III, in a Feb. 11 memorandum for the president, said the pending bill is “contrary to public health goals and to the administration’s established position to protect the Filipinos, especially the youth, from the harms associated with the use of and exposure to smoke and emissions from tobacco products, vapor products, and heated tobacco products.”
President Rodrigo Duterte has the option to veto the vape bill, allow it to lapse into law 30 days from receipt, or send it back to Congress with his veto message.
Take a look at the different provisions here and how they could impact on public health, according to health experts:
This story is part of the project Seeing Through the Smoke, which is supported by a grant from the International Union of Tuberculosis and Lung Disease (The Union) on behalf of STOP, a global tobacco industry watchdog.