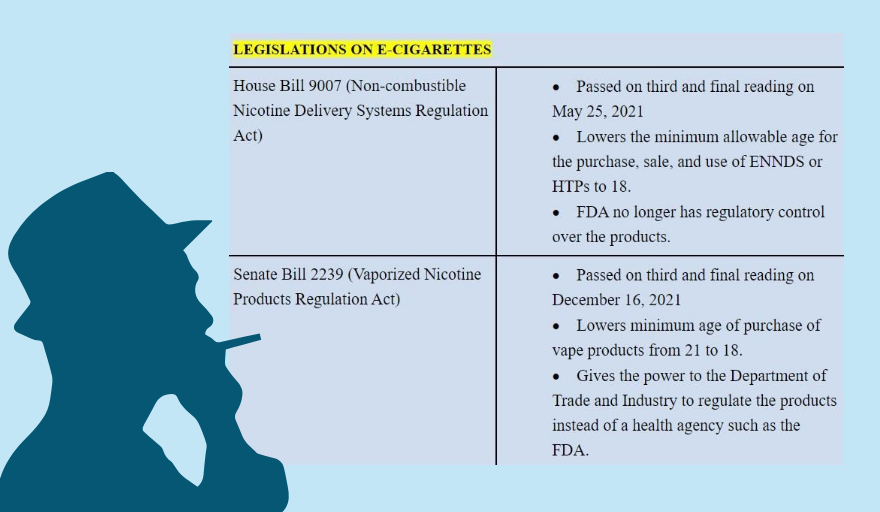
MANILA — It won’t be long before 18-year-olds will be legally allowed to purchase vape products on their own. On December 16, 2021, the Senate passed Senate Bill 2239, or the Vaporized Nicotine Products Regulation Act, which lowers the minimum allowable age for the purchase of vape products from 21 to 18.
It’s the setback that health and tobacco control advocates had been dreading ever since the Lower House passed a similar bill (House Bill 9007, or the Non-Combustible Nicotine Delivery Systems Regulation Act) on May 25, 2021.
Once enacted, both laws will also transfer the responsibility of regulating these products from the Food and Drug Administration to a non-health agency, the Department of Trade and Industry. This is something that has stunned health advocates though they may have already seen this coming.
A history of animosity
“Is the FDA not happy with the funds provided by the government in discharging its regulatory function?” an irked Representative Rodante Marcoleta asked an official of the agency in a Congressional hearing one morning in June 2021.
Marcoleta and other members of the House of Representatives’ committee on good government and public accountability are grilling the Food and Drug Administration (FDA) over its “questionable receipt of private funding” from international groups that, they said, could be tantamount to “foreign interference” that “undermined” the country’s sovereignty.
The hearing was initiated by Deputy Speaker Deogracias Victor “DV” Savellano, representative of Ilocos Sur, the province where Candon City, the “Tobacco Capital of the Philippines,” is located.
For over three hours, lawmakers lashed out at officials of the FDA, pressuring them to admit “conflict of interest” in accepting grants from the Bloomberg Initiative to Reduce Tobacco Use (Bloomberg Foundation) and the International Union Against Tuberculosis and Lung Disease (The Union).
FDA director Ana Rivera justified their grant-seeking venture by revealing that the agency had little allocation in 2016, and so funding research was nearly impossible.
“In 2016, the funds allotted to our office was only P4 million for one year and this would cover all operational expenses so at that time we don’t have extra funds to conduct research and hire personnel,” Rivera told lawmakers.
The grant from The Union and Bloomberg Initiative amounting to over $150,000, or roughly P7 million, was used by the FDA to hire additional research personnel, pay for operational costs, and purchase equipment for its research projects, Rivera added.
The Union and Bloomberg Initiative support projects that develop “high-impact, evidence-based tobacco control interventions,” the FDA adds.
According to Cagayan de Oro Rep. Rufus Rodriguez, however, the FDA, in accepting the foreign grants, may have violated three laws:
• Section 7 of Republic Act 6713 or the Code of Conduct and Ethical Standards for Public Officials and Employees for “soliciting and acceptance of gifts.”
• Section 11 paragraphs 1 and 3 of Batas Pambansa Blg. 39 or the Foreign Agents Act of 1979 “where it is prohibited for foreign agents and foreign principals to request any information or advice from agency or officials of Philippines pertaining to the foreign or domestic policies, and for Philippines officials to act as agents of foreign principal.”
• Sec 3-E of Republic Act 3019 or the Anti-Graft and Corrupt Practices Act “which prohibits giving any private party any unwarranted benefits.”
This, even as FDA had earlier cited Section 18 of RA 9711 (FDA Act of 2009), along with its Implementing Rules and Regulations (IRR) which allows them, through their special regulatory fund, to accept grants, donations, and all other endowments from local and external sources in accordance with pertinent laws, rules, and regulations.
“Did you not see that there was a connection between the donation and the execution of the act of the FDA in regulating this particular industry?” Rodriguez asked.
Rivera, who oversaw the project involving the grants, said nothing in their memorandum of agreement (MOA) gave the Bloomberg and Union any control on FDA.
“In the MOA signed, there was no exact provision that they would have oversight or (that) they will contribute to the policies of FDA,” she told Rodriguez.
She added that all their policies on tobacco are guided by the World Health Organization’s (WHO) Framework Convention on Tobacco Control, of which the Philippines is a signatory.
“This remains our guiding principle in the development of policies and guidelines within the FDA,” Rivera said.
But Rodriguez was somehow of the impression that FDA has no agency in deciding tobacco-related policies, insisting it was influenced by the Bloomberg and Union.
“No person giving something would put that in fine print, but of course the FDA is very knowledgeable that the Bloomberg and the Union have advocacies and certainly that affects your decision-making in regulations pertaining to tobacco,” Rodriguez said, asserting that this effectively undermines FDA’s ability to independently craft policies.
Rejecting the insinuation that the FDA was “influenced,” Rivera insisted that the advocacies of the grant-giving bodies are aligned with theirs.
“As a public health agency, our advocacy is really against tobacco. So, we have a tobacco control program … it is the whole of the DOH (Department of Health) and FDA that has this advocacy, to at least reduce the tobacco-related diseases,” Rivera said.
In a bizarre turn, Rodriguez accused FDA of “waging a war against the tobacco industry.”
“I’m surprised that the FDA is waging a war against the tobacco industry… Why are you against a legitimate industry?”
Puwersa ng Bayaning Atleta partylist Rep. Jericho Nograles also castigated the FDA for “misdirecting” the Congressional hearing when it allegedly claimed earlier that it did not receive foreign funding.
For the duration of the hours-long hearing, lawmakers took turns at interrogating FDA over the controversial funding.
While the behavior of lawmakers and the direction of the whole hearing were shocking to casual observers, health advocates are convinced it was just part of a larger, coordinated, and more sinister global operation.
But who is behind it?
Where it all began
To understand the motives behind the “absurd” Congressional hearings, longtime tobacco control advocate Dr. Ulysses Dorotheo says people need to look into the series of exposés by Reuters dubbed as “The Philip Morris Files: The secrets of the world’s biggest tobacco company.”
Published in 2017, these investigative reports uncovered internal documents from Philip Morris, including a damning slide presentation by corporate affairs from 2014 in which they identified “anti-tobacco extremists” that needed to be opposed.
“They already identified they needed to set up opposition against particular groups and that included the Bloomberg Foundation, the Bill and Melinda Gates Foundation, the WHO (World Health Organization), even SEATCA (Southeast Asia Tobacco Control Alliance) as a regional organization,” according to Dorotheo, who is executive director of SEATCA.
Dorotheo says that plan is being carried out now.
One of its manifestations, Dorotheo points out, was the inception of some “health groups” that, in one way or another, have links to the tobacco industry.
“There are regional groups now like the Coalition of Asia Pacific Tobacco Harm Reduction Advocates (CAPHRA). They are a member of the International Network of Nicotine Consumer Organisations (INNCO), and INNCO has a grant from the Foundation for a Smoke-Free World (FSFW), which is fully funded by Philip Morris. I think the current executive director of INNCO used to work for the FSFW,” Dorotheo says.
Dorotheo is referring to incumbent INNCO executive director Charles Gardner, who used to work as FSFW’s director of health science and technology.
How does this all tie into the House hearings? Dorotheo says one must look at the bills in Congress to realize what is at stake.
“If you look at the recent social media posts of CAPHRA, they [said] that the pending legislation in Congress regarding ENDS/ENNDS (electronic nicotine and non-nicotine delivery systems) and HTPs (heated tobacco products) will be the most progressive daw. And it will lay a foundation for other countries who will look at this and say, ‘Oh the Philippines has done it, we should follow.’”
Demonizing the FDA
By May of 2022, the Philippines will implement the regulatory provisions of Republic Acts 11346 and 11467, giving the FDA the power to govern the usage and sale of HTPs and other vapor products.
The IRR of the two laws contain highly contested provisions wildly opposed by the tobacco industry, among them the banning the sale of HTPs to anyone below 21 years old and the restriction to produce flavors appealing to the youth.
However, House Bill 9007, which was approved on third and final reading in March, is threatening to prevent the enforcement, and eventually invalidate, the good intentions of the IRR.
“It will reverse all the [gains from the IRR]. The Department of Trade and Industry (DTI) will be the lead regulating agency [for HTPs and ENDS], the minimum age will be brought down to 18, many flavors will be allowed, including fruity flavors, and online advertising will also be allowed… They’re doing this before the effectivity of the existing legislation can come into force,” Dorotheo warns.
Dorotheo, who followed how the Congressional hearings on House Bill 9007 unfolded, says there seemed to be a sense of urgency in approving it, despite opposition from several health groups, advocates, and even government agencies.
“Talagang minadali (It was fast-tracked) … If you look at the final bill that was passed in Congress, totally ignored yung public health side eh (the public health side was totally ignored) — the DOH, FDA, WHO, all the NGOs,” he laments.
Based on published news reports, the committee endorsed its final report for plenary debates on March 16. The bill was passed on second reading on May 19, and the House approved it on third and final reading six days later on May 25.
Several lawmakers and health advocates criticized many provisions in the approved House bill, claiming it diluted the spirit of the proposed regulation.
Rep. Sarah Elago of the Kabataan Party-list said they strongly oppose “… the lowering of the minimum legal age of access from 21 to 18 years old. This youth representation proposes that the age of access restriction be raised to 25 years instead, in light of new studies and evidence which suggest that young persons below the age of 25 are highly likely to retain nicotine addiction.”
“House Bill No. 9007 is a complete departure from the regulatory framework, protection of children and young people and certain health safeguards already in place in RA 11467 that we ourselves passed only very recently,” Agusan del Norte Rep. Lawrence Fortun lamented.
(Republic Act 11467 amends certain parts of the Internal Revenue Code, or RA 8424, increasing the excise taxes on, among others, e-cigarettes and heated tobacco products.)
Quezon Rep. Angelina Helen Tan, House Committee on Health chair, said the bill “pretends to be a health measure for all but only gives primordial consideration to trade and commercial interests of the few.”
Speaking to CNN Philippines after the passage, HealthJustice Philippines legal consultant Atty. Ben Nisperos said House Bill 9007 is a “betrayal of public health.”
“We are very much disappointed because we consider this as a retrogression of our strict policies related to tobacco products… We consider this as a betrayal of public health because the provisions of the new bill are less strict and weaker than our present regulation,” Nisperos said.
As Senate Bill 2239 was passed just a few months later, health advocates expressed their dismay at the bill’s passing, saying it is, once again, a “betrayal of the Filipino people.”
For Dorotheo, the House hearing on FDA’s alleged “violation” in accepting the Bloomberg and Union grants is targeted to discredit the organization.
“The hearing on The Union funding is, in a way, a smokescreen… to attack the FDA — that it’s not a credible regulatory agency. They’re saying they can’t let the FDA regulate it, [that] it should be the DTI — yun yung magiging basis nila (that will be their basis),” Dorotheo says.
Industry puppets?
Analyzing the annual report of Philip Morris in the United States, Dorotheo and his group in SEATCA found something curious.
“We found that the amount of [money] for CSR, charitable activities that went to the Philippines last year (2020) increased. In 2019, they donated about $2.9 million, most of this is through Jaime V. Ongpin Foundation. And then in 2020 it became $13.4 million. Obviously, these are for respirators, PPEs [personal protective equipment], things like that, because of the pandemic,” Dorotheo said.
A series of Eco-Business exposés published earlier this year uncovered the beneficiaries of Jaime V. Ongpin (JVO) Foundation’s pandemic donations, which included the City of Cagayan de Oro, represented in Congress by Rodriguez, the lawmaker leading what observers described as “character assault” against the FDA.
But Rodriguez’s jurisdiction was not the only beneficiary of the JVO Foundation’s donations.
Also recipients of the foundation’s pandemic aids are organizations and jurisdictions linked to Nograles, Marcoleta, Savellano, and Nueva Ecija Rep. Estrellita Suansing, who earlier accused the FDA of “premature” and “bad faith” in relation to its issuance of regulations governing vapor and heated tobacco products.
“Where else in the world would you have legislators attacking regulatory agencies for doing their job?” an incredulous Dorotheo said.
Philippines, the battleground
The alleged attacks orchestrated by the tobacco industry are “more ferocious than before,” according to Dorotheo, mainly because of the magnitude of the [bills] in Congress, which would set a precedent for other countries’ regulation on novel tobacco products.
“They know that… it would give them a way to show that other countries should follow — a domino effect is what they’re expecting to happen,” he says.
The Philippines is their laboratory rat, and the results of their experiment will be waved before the international community as an “example” of success.
“The Philippines is their current battleground. If they win this, it will open up opportunities for them in other countries,” Dorotheo says.
As the tobacco industry reaches its goal, Dorotheo proposes a simple counter narrative.
“The only way to battle falsehood is with the truth.”
Sought for comments, FDA says they are unbothered by the attacks against them.
“We are doing our jobs as mandated,” asserts FDA director-general Eric Domingo, “and we do not allow this to distract us from our work to protect public health.”
This story is produced under the Nagbabagang Kuwento (Burning Stories) Tobacco Control Media Program of the Probe Media Foundation Inc. supported by the Campaign for Tobacco-Free Kids.