If President Rodrigo Duterte approves the vape bill before he leaves Malacanang on June 30, the Department of Health (DOH) says it will “result in an absurd situation” wherein similar products that cause harm and danger are regulated differently.
The DOH renewed its call on the president to veto the bill, which strips the Food and Drug Administration (FDA) of the mandate to regulate novel tobacco products, including vapes and heated tobacco products (HTP).
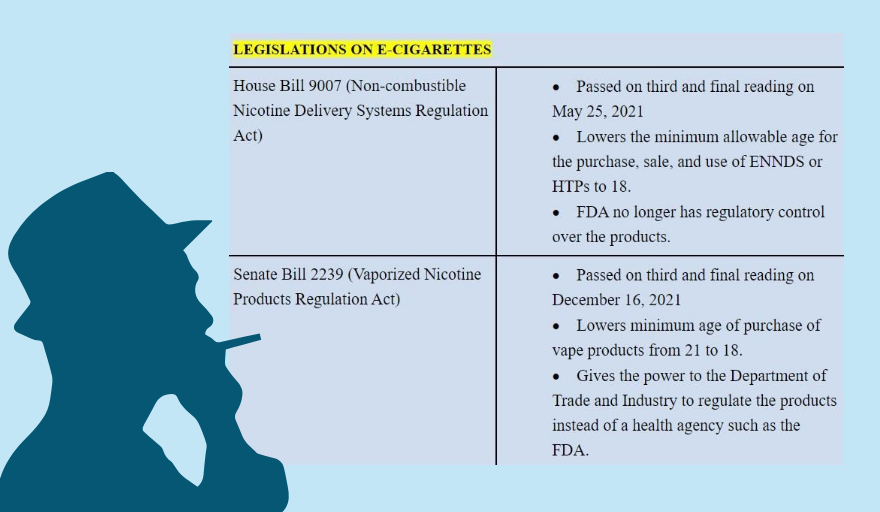
Section 21 of the proposed Vaporized Nicotine and Non-Nicotine Productions Regulation Act transfers the authority to regulate such products to the Department of Trade and Industry (DTI). “Once the bill becomes law, vapes will be regulated not as a health product, but as a consumer product, which makes it more dangerous for the public,” the DOH said on its official Viber account.
The controversial measure was transmitted to Malacañang on June 24, just six days before the president’s term ends on June 30. Duterte now has the option to veto or sign it into law, or let it lapse into law after 30 days from submission.
“The transmittal of the bill at this late hour is a devious attempt to evade scrutiny by the outgoing administration, and to get the bill passed on to the next administration,” said Sen. Pia Cayetano, who opposed the measure from the outset.
Congress ratified on Jan. 26 a version of the vape bill that lowers the minimum age of restriction to vapes and HTPs from 21 to 18 years old and allows more flavors, instead of just two (plain tobacco and menthol), as mandated in an existing law.
(Read Vape bill version 2022: Congress ‘hijacks’ stringent regulations)
Just last June 13, the Supreme Court (SC) ruled in favor of the FDA, stating that the agency has “competence and mandate to ensure safety, efficacy, purity, and quality of health products,” including tobacco products.
The High Court overturned a 2012 decision by the Regional Trial Court of Las Piñas, Branch 255, granting the petition of the Philippine Tobacco Industry (PTI) to prohibit the enforcement of the implementing rules and regulations of Republic Act (RA) 9711 or the Food and Drug Administration Act of 2009.
RA 9711 granted FDA regulatory authority over “all health products.” PTI then argued that the law excludes tobacco products, following Sec. 25 of RA 9211 or the Tobacco Regulation Act of 2003 that granted the Inter-Agency Committee Tobacco (IAC-T) the “exclusive jurisdiction over tobacco products, including its health aspect.”
The DTI heads IAC-T, while the DOH sits as vice chair.
In a 37-page decision, Justice Marvic Leonen wrote that such provision “does not automatically place tobacco products outside the [FDA’s] regulatory authority.”
Leonen said the IAC-T has “limited jurisdiction over tobacco products and does not regulate all their aspects,” noting that RA 9211 includes the regulation of distribution, access, sale, labeling, advertisements, sponsorships, and promotions of tobacco products and not the health aspects.
The senior associate justice said the respondents, who are representing major transnational tobacco companies in the country, “proposes an interpretation of our law that will effectively remove them” from the regulation of DOH and FDA.
“This not only leads to an absurd result, but it is also contrary to law and our international obligations,” he added, referring to the Philippines’ commitment as a signatory to the World Health Organization Framework Convention on Tobacco Control (WHO FCTC).
While the SC decision did not cover novel tobacco products, the DOH said it “immortalizes the fact that tobacco products and all related products, such as [HTPs], vapor products, and other emerging similar products are health products and should be regulated under the FDA.”
Several health experts have repeatedly noted that vapes and HTPs pose threats to human health. “Reduced risk” claims are misleading, according to experts, because these are based on short-term evidence and long-term studies are needed to assess significant harms.
The DOH hopes that the outgoing president will stick to his earlier anti-smoking pronouncements and will continue to prioritize health over the tobacco industry’s interests.