Rolling out a vaccine that works safely for millions around the world is the huge mantle biopharmaceutical and research experts have taken upon themselves over the last ten months since the coronavirus disease 2019 (COVID-19) outbreak.
In an online forum on Oct. 26, Secretary Fortunato de la Peña of the Department of Science and Technology (DOST), Dr. Beaver Tamesis of Merck Sharp & Dohme (MSD) Philippines, and Dr. Jean-Antoine Zinsou of Sanofi Pasteur Philippines said the target availability of a COVID-19 vaccine by mid-2021 is still “very optimistic” even with the accelerated process for clinical trials.
“My own estimate is if for example we can start the trials itself even at the start of December or late November—because they say the trials will be anywhere between three to six months, that will already be the compressed time—we are looking at the end of the trials at the second quarter of 2021,” the DOST chief said at the forum organized by the Foreign Correspondents Association of the Philippines (FOCAP).
But de la Peña adds that actual mass vaccination may take some time. Tamesis also said he suspects that Filipinos may “have to live with our current normal for the full of 2021.”
Three vaccine developers already filed independent applications with the Philippines’ Food and Drug Administration (FDA) to conduct Phase 3 clinical trials. These are Sinovac Biotech’s CoronaVac from China, Gamaleya Research Institute’s Sputnik V from Russia, and Johnson and Johnson’s Janssen COVID-19 vaccine from the United States.

The Department of Science and Technology (DOST) and Philippine offices of pharmaceutical companies Sanofi Pasteur and MSD joined the Foreign Correspondents Association of the Philippines (FOCAP) on Oct. 26 in an online forum to discuss the current state of vaccines for COVID-19. Screengrab from FOCAP’s Facebook Live video
The World Health Organization (WHO) Solidarity Trials, meanwhile, moved the schedule for its vaccine candidates in the Philippines under the COVID-19 Vaccine Global Access Facility, or COVAX, from-end October to December 2020, according to de la Peña.
COVAX is a collaborative initiative led by WHO, Gavi, the Vaccine Alliance, and the Coalition for Epidemic Preparedness Innovations (CEPI) that seeks to manufacture a diverse vaccine portfolio accessible to everyone.
The Facility aims to procure two billion doses by the end of 2021 to be distributed among 172 countries to immunize 20 percent of their population. Lower to middle income countries will also receive financial assistance from the Gavi COVAX Advance Market Commitment (AMC), who are now pegging the price of two vaccine doses at 21 U.S. dollars or approximately P1,000.
De la Peña said among those who will receive the first doses of a “successful vaccine” are the vulnerable and exposed, such as health workers and frontliners.
Not skipping steps
There are a number of vaccine candidates to fight SARS-CoV-2 being developed. The Vaccine Centre at the London School of Hygiene and Tropical Medicine, which has built a COVID-19 vaccine tracker, has recorded 248 candidates in the pipeline as of Oct. 26.
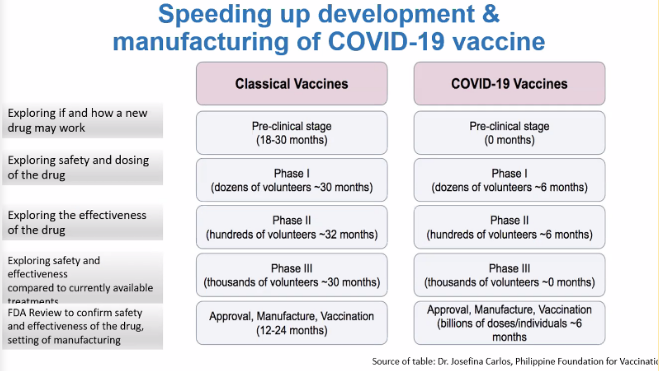
According to Tamesis, vaccine development that used to take years has now sped up to mere months in response to the pandemic. Despite the unusual speed, pharmaceutical companies said they are not leaving out any steps in the process.
Dr. Beaver Tamesis, country president of MSD, compares the timelines for development and manufacturing of classical vaccines with COVID-19 vaccines. Screengrab from FOCAP’s Facebook Live video
Of the vaccine candidates recorded by the Vaccine Centre, 197 are in pre-clinical trial stage while 51 have advanced to various phases of clinical testing.
In pre-clinical trials, vaccines are tested in animals such as mice and monkeys to see if it produces an immune response. A vaccine candidate then goes through three phases that vary in the number of test volunteers and objectives: 10 to 100 people in Phase 1 to test safety, 100 to 1,000 in Phase 2 to determine right dosage, and over a thousand people in Phase 3 to confirm its efficacy and safety to humans when compared to other treatments.
“Not because we are going faster, that we are neglecting some aspect of the development of this vaccine especially when it comes to the safety of the vaccine,” said Zinsou, general manager of Sanofi Pasteur Philippines.
Testing for efficacy in Phase 3 means that two populationsーone receiving the vaccine, the other not receiving itーare compared to define the “statistical reliability” of the study based on the parameters of the disease in a given epidemiological context, he added.
Diversity of the populations enrolled in the clinical studies can be a factor that determines speed and scale, according to MSD’s Tamesis, who is also the president of the Pharmaceutical Healthcare Association of the Philippines (PHAP). A site may have a narrower vaccine protocol that works only for a particular segment of the population and therefore, can get immediate regulatory approval, he added.
Chinese Sinovac’s CoronaVac, for example, focused on adults between the age of 60 and 89 in its first and second phases of trials, while Russia’s Gamaleya Sputnik V had a wider age range of 18 to 59 years.
“Pharmaceutical companies could do secondary follow-up studies in older, more complex patients just so they can expand the potential recipients of that vaccine, so that’s how we normally do things, pre-pandemic,” Tamesis said.
During the pandemic, as the speed and magnitude of Phase 3 studies will include tens of thousands of people, governments should monitor the administration of the vaccines to ensure that every rare adverse event is identified, said Zinsou.
In the Vaccine Trials Primer by the Department of Health (DOH), DOST, the DOST-Philippine Center for Health Research and Development (PCHRD), and FDA, the Philippines has established the Vaccine Expert Panel, a group of technical experts and scientists, and an Ethics Review Board to ensure vaccine safety before any clinical trial is allowed to be conducted in the country.
“We can trust in the system so long as they go through the different phases and they actually have the right number of patients enrolled in these kinds of trials,” Tamesis told reporters in the forum.
Although side effects may also occur in the investigational vaccines such as pain, redness, itchiness, or swelling at the injection site, as well as fever, fatigue, headache, dizziness, diarrhea, and nausea, these are documented and managed by the independent monitoring committee.
What this means is that overall effectiveness of the vaccine also depends on regulatory authorities’ ability to review data from all phases and on post-marketing surveillance.
Vaccines are not be all, end all
Experts, however, highlighted that having a vaccine available is not a be all, end all solution. Although a COVID-19 vaccine is yet to be meted out, hygienic measures such as wearing of face masks, frequent handwashing, and physical distancing will carry on in the new normal.
The national immunization program of the DOH also has to get all stakeholders engaged, said Tamesis. This involves getting doctors, parents, teachers, and city officials to “influence the acceptance” of vaccines from DOH.
Vaccine hesitancy remains a concern in the country following the Dengvaxia controversy in 2017, said Zinsou, wherein parents whose children had received the dengue vaccine were alarmed by its reported adverse effects.
In a WHO research from April to June 2020, it was found that the respondents from two highly urbanized communities in Manila refused at least one vaccination for their children because of the “negative media information” and “concerns about vaccine safety and their side effects,” relating to the Dengvaxia vaccine.
Tamesis said that constant research on treatment options should also continue to anticipate the refusal by some people of a vaccine. “Not everyone will take up the vaccine. There will always be those who will refuse.”
Given this, Zinsou also urges awareness campaigns to fight mis- and disinformation that may contribute to vaccine confidence.
“It’s very important that we do not stand still waiting for the vaccine to arrive. We have a lot of work to do to get prepared,” he said. “We want to raise the awareness and make sure that people accept that we will prioritize the administration of this vaccine.”
As of Oct. 27, the Philippines has a total of 373,144 confirmed cases with 37,489 active, 328,602 recoveries, and 7,053 deaths, according to the DOH.
(This story was produced under a Health Fellowship program carried out by VERA Files with support from Facebook. Find out more about this project here.)