President Rodrigo Duterte’s wish that, by December, the Philippines will be COVID 19-free with the help of Russia’s novel coronavirus vaccine may be delayed.
Food and Drug Administration (FDA) Director General Eric Domingo confirmed in an Aug. 13 interview with ANC that the administration requires a vaccine to complete its phase 3 clinical trials before it could be registered and approved for distribution. (See VERA FILES FACT SHEET: Five questions on COVID-19 vaccines, answered)
This means it should have “been tried on several thousand patients,” found to have “very minimal or no serious side effects,” and proven “effective in inducing an immune response.”
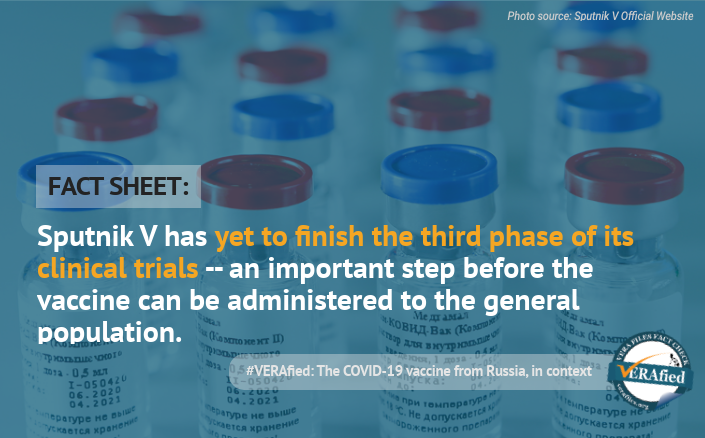
Sputnik V, the COVID-19 vaccine dubbed by Russian President Vladimir Putin as the “world’s first”, has yet to finish the third phase of its clinical trials — an important step before it can be administered to the general population.
Presidential Spokesperson Harry Roque confirmed Aug. 12 that the country will take part in the drug’s phase 3 trials, to be simultaneously held in Russia and the Philippines from October 2020 to March 2021.
Phase 3 studies are “often the step right before a new treatment is approved,” according to a World Health Organization (WHO) page on clinical trials. Russia bypassed this usual order of procedure and instead registered its vaccine first before testing it for the third phase.
Dr. Jaime Montoya, Executive Director of the Philippine Council for Health Research and Development, said in an Aug. 13 interview with CNN Philippines that after the clinical trials — expected to end early January 2021 — manufacturers of the vaccine will analyze and submit a report to the Russian authorities. Once approved, the report will then be submitted to regulatory authorities of other countries, including the Philippines’ FDA.
FDA’s review of the results is estimated to go on for one to two months. Following this timeline, Montoya said the vaccine could be approved by around April 2021, assuming it passed FDA’s thorough review. It is only then that the vaccine can be sold in the Philippine market.
Around 1,000 healthy, randomly selected Filipinos will participate in said trials. Individuals aged 18 to 59 years old will be the prioritized participants of the tests, according to Dr. Nina Gloriani, professor in the University of the Philippines Manila College of Public Health and member of the country’s sub-Technical Working Group for vaccine trials.
However, WHO’s Aug. 10 list of all COVID-19 candidate vaccines show that Sputnik-V, developed by the Gamaleya Institute in Russia is still in the first stage of clinical evaluations.
Of the 28 candidate vaccines worldwide that are already being tested clinically, only six are in their third phase of trials, including vaccines being developed in the United Kingdom, China, and the United States.
Skepticism surrounding the vaccine abounds, with reports of Russia possibly “cutting corners” in order to lead the world’s COVID-19 vaccine development.
This, fueled by the absence of publicly available data on Sputnik-V, raises questions about its effectiveness and safety.
WHO, for its part, said it is “in close contact with Russian health authorities” in discussing the prequalification process for the vaccine. This involves going through “a rigorous review and assessment” of all data gathered from its clinical trials.
Last week, WHO spokesperson Christian Lindmeier also said any vaccine “should be […] going through all the various trials and tests before being licensed for roll-out,” as quoted in media reports.
This statement came after Russia’s Industry and Trade Minister Denis Manturov said that the country is targeting serial production of the vaccine “as early as in September,” according to state media TASS on Aug. 3.
Two days earlier, state-run news agency RIA Novosti cited Russian Health Minister Mikhail Murashko in pronouncing that mass vaccination in the country had been planned for October.
Sources
Inquirer.net, Palace: Duterte willing to be ‘guinea pig’ for Russia vaccine, but it depends on PSG, Aug. 12, 2020
Manila Bulletin, Palace: Duterte willing to be a guinea pig for Russia’s COVID-19 vaccine if PSG allows it, Aug. 12, 2020
The Times of Israel, Philippines’ Duterte says he’ll be Russia vaccine ‘guinea pig’, Aug. 12, 2020
The Business Times, Philippines’ Duterte will be Russia vaccine ‘guinea pig’ as talks begin, Aug. 12, 2020
Presidential Communications Operations Office, Interview with Presidential Spokesperson Harry Roque by Pinky Webb (CNN Philippines – The Source), Aug. 12, 2020
Presidential Communications Operations Office, Talk to the People of President Rodrigo Roa Duterte on Coronavirus Disease 2019 (COVID-19), Aug. 11, 2020
Russian Direct Investment Fund Youtube channel, Meeting of the President of Russia with the Government members, 11.08.20, Russia-24, Aug. 11, 2020
Presidential Communications Operations Office, Press Briefing of Presidential Spokesperson Harry Roque, Aug. 13, 2020
CNN Philippines Youtube channel, DOST: Meeting with vaccine manufacturer productive, Aug. 13, 2020
Department of Health, WATCH: DOH Presscon, July 8, 2020
ABS-CBN News Youtube channel, Philippines to be ‘very careful’ in potential Russian vaccine trial: FDA, Aug. 13, 2020
World Health Organization, Clinical trials, n.d.
World Health Organization, DRAFT landscape of COVID-19 candidate vaccines, Aug. 10, 2020
CNN Philippines, Putin says Russia has approved ‘world first’ Covid-19 vaccine. But questions over its safety remain, Aug. 11, 2020
The New York Times, Russia Approves Coronavirus Vaccine Before Completing Tests, Aug. 11, 2020
The Washington Post, Russia unveils coronavirus vaccine ‘Sputnik V,’ claiming breakthrough in global race before final testing complete, Aug. 12, 2020
Reuters, WHO says discussing new COVID-19 vaccine with Russia, Aug. 11, 2020
The Independent, Coronavirus: Philippine president Duterte offers to be ‘injected in public’ with Russian vaccine to dispel safety concerns, Aug. 11, 2020
The Jakarta Post, WHO urges Russia to follow guidelines on virus vaccine, Aug. 5, 2020
TASS, Three Russian firms to start serial production of COVID-19 vaccine in September, Aug. 3, 2020
RIA Novosti, В России завершились клинические испытания вакцины от коронавируса, Aug. 1, 2020
(Guided by the code of principles of the International Fact-Checking Network at Poynter, VERA Files tracks the false claims, flip-flops, misleading statements of public officials and figures, and debunks them with factual evidence. Find out more about this initiative and our methodology.)