Early this month, a study went up on the pre-print server bioRxiv. In it, the research team builds the case for “the emergence of a more transmissible form” of SARS-CoV-2, the virus behind the COVID-19 pandemic. Mutations, essentially tiny changes to the virus’ genetic code, may reflect on the overall structure of the spike protein.
In turn, they argue, these may somehow make the spike a better key, able to more efficiently get the virus inside its target host.
The conclusions of the study shouldn’t be taken as final, at least not yet. Pre-prints, after all, are manuscripts that have yet to pass through the rigors of academic peer review, and are pending publication in scientific journals.
Nevertheless, the media took the soundbite and ran with it. Not long after, the internet was awash with alarming headlines. One writes that the virus “has mutated and appears to be more contagious now,” while another claims that “a now-dominant strain of the coronavirus could be more contagious than original.”
Could it be true that the world is now facing a supercharged strain of SARS-CoV-2?
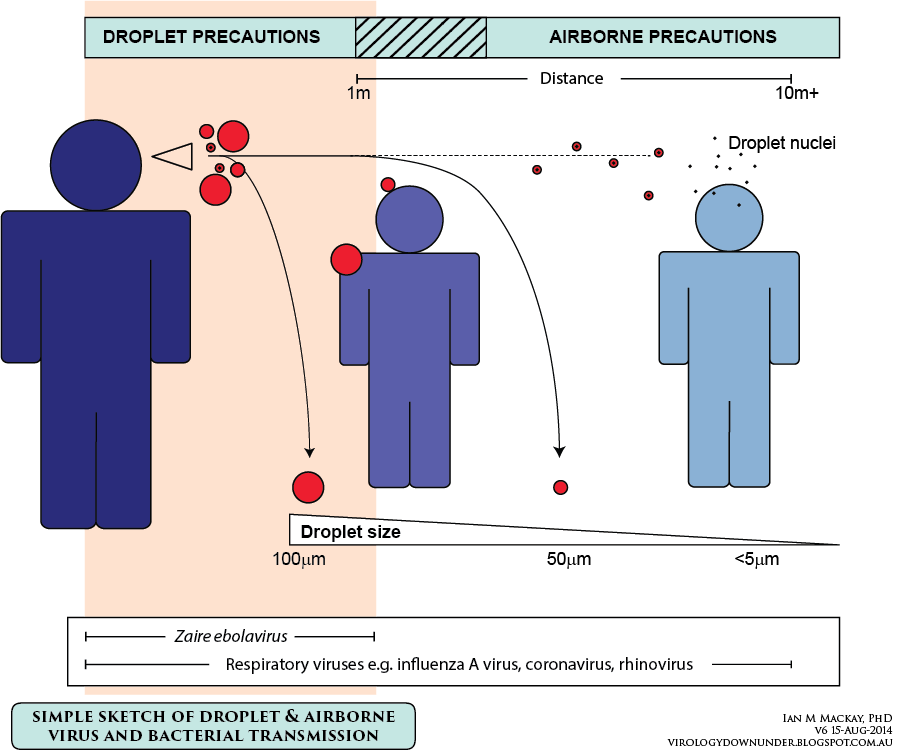
Illustration from virologydownunder.com
“Coronaviruses like SARS-CoV-2 are mainly transmitted to susceptible individuals through infectious respiratory droplets,” according to Mary Grace Dacuma, PhD, a molecular epidemiologist specializing in Infectious and Tropical Diseases, from the Institute of Biological Sciences, University of the Philippines Los Baños.
“Respiratory droplets are greater than five to 10 micrometers in diameter and travel less than a meter. Any susceptible person who is less than a meter from an infected person can acquire the infection from respiratory droplets through the latter’s sneezing, coughing, or talking,” she says.
Five micrometers is tiny, far smaller than what the naked eye can see. The infected person’s sneeze or cough expels dozens, if not hundreds, of these droplets, putting around two people near them at risk, at least according to early estimates of SARS-CoV-2’s basic reproduction number, or Rₒ. (The actual value is still contentious and is likely to be between one and seven new infections every four days or so).
Of course, in practice, this is not a static figure. The average number of possible infections changes according to circumstance. For example, at the height of the outbreak in Wuhan, the pandemic epicenter, one study reported that each sick individual would go on to infect almost four other people.
After the dramatic mobility control measures had taken effect, this number dropped to below one, marking the outbreak’s denouement.
At present, Dr Dacuma continues, there is no consensus as to whether or not the virus is airborne. But it’s definitely a possibility, especially in hospitals, where affirmative evidence is slowly mounting.
Leslie Dalmacio, PhD, President of the Philippine Society of Biochemistry and Molecular Biology, and a professor at the UP College of Medicine, agrees. “There is no solid evidence yet that it is airborne,” she says. “It would be more problematic if it were airborne because it would be able to infect more people.”
It’s easy to stay away from someone that’s obviously sick. More and more, however, experts are seeing that even people who have the virus but seem fine may pass it on. This is a crucial distinction between SARS-CoV-2 and SARS-CoV-1, its predecessor from the early 2000s and culprit behind the outbreak of severe acute respiratory syndrome, or SARS.
Once inside the body, the SARS virus makes a beeline for the lungs where, over the next week or two, it explodes in number. Recent evidence suggest that SARS-CoV-2 doesn’t. Instead, it lingers unfelt in the throat. In under five days, the virus will have multiplied enough to make the patient infectious; towards the end of the disease, transmission appears to be very low. Unlike SARS, COVID-19 doesn’t take its time.
While a couple of small-scale, disjointed studies hardly qualify as a smoking gun, the findings, if true, fly in the face of prevailing control measures. Screening, testing, and isolating people with signs of COVID-19 may not cut it because the window to stop them from spreading the disease may have already closed.
It remains unclear why SARS-CoV-2 does this, though it’s likely that genetic mutations are at least partly responsible. But to definitively conclude that mutations cause this change in behavior―or lead to a more efficient spike protein―will require much more experimentation and evidence.
Mutations sound scarier than they really are. In the most basic sense, mutations pertain to permanent changes in the genetic sequence. Sometimes, as in some very aggressive breast cancers, these can lead to profound consequences on public health. But the vast majority of mutations stay silent and will go unnoticed. Others, still, prove detrimental to the organism and are edged out by natural selection.
Some mutations are triggered by environmental exposures, such as to ultraviolet radiation and to particularly strong chemicals, which can reach deep inside cells and mess with the genetic material. Others arise spontaneously, as products of a faulty replication machinery that fails to copy the genetic material perfectly.
In viruses, especially in RNA viruses like SARS-CoV-2, this process is notoriously sloppy. As a result, mutations, tiny errors in the genetic code, are not only normal, but expected.
This is why several other papers have also found mutations in the SARS-CoV-2 genome that, conceivably, could make it a more worrisome threat, as if it isn’t bad enough already. What’s lacking, however, is hard, conclusive evidence of such effects.
By and large, the authors of these papers themselves admit that, if anything, their findings underline the need for future, more aptly designed studies. One group writes that “further experiments are needed to determine the functional consequences” of the particular mutation they spotted.
Typically, these endeavors will entail well-controlled lab experiments that explicitly show, either in cells or in animal models, that one variant indeed trumps the other, be it in terms of faster replication, of easier virus-host binding, of stronger resistance to drugs, or of better overall fitness.
And even then, these results will still need to go through a slow and iterative process of verification and replication by other different labs before they pass as consensus. Good science, after all, takes time.
All of this, of course, is not to say that SARS-CoV-2 isn’t evolving. It is. All viruses do. But does this mean the world is now dealing with some eight different strains? Highly unlikely. A mutation does not a strain make.
But gradually and over time, it might. As the genetic mismatches pile up, a new and functionally distinct lineage of SARS-CoV-2 may emerge and branch off. This new group needs to be demonstrably different in significant ways―in how it jumps from person to person, or in how it leads to sickness, or in how it responds to treatment.
This is what qualifies as a strain and is the reason why flu shots have to be updated yearly. The influenza virus changes at such a rapid pace that it renders last year’s vaccines ineffective against this year’s strain.
Fortunately, this shouldn’t be an urgent problem for COVID-19. While there’s still no consensus for SARS-CoV-2 specifically, coronaviruses, as a family, are relatively stable, genetically speaking.
According to early projections of Nextstrain, an online, open-source platform that provides “a real-time snapshot of evolving pathogen populations,” SARS-CoV-2 accrues around 25 substitutions per year. These refer to the mutations that stick and, despite the pressures of natural selection, persist in distinct lineages. The Nextstrain estimates were drawn using over 5,000 SARS-CoV-2 genome sequences that have been published online.
For context, the seasonal flu mutates around two to four times faster.
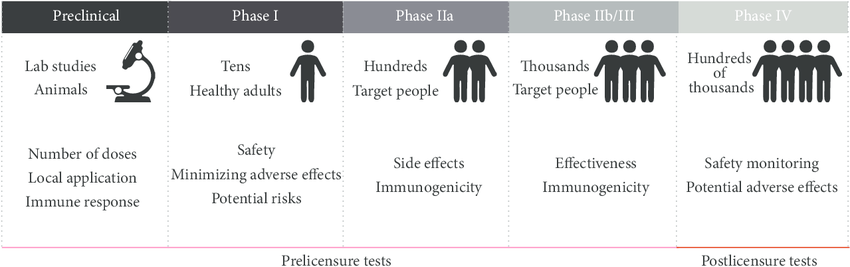
Illustration from www.researchgate.net
This could mean that vaccines, once available, may confer lasting protection against SARS-CoV-2. It might take a while for us to know for sure, though, because while more than a hundred are in the pipeline, most are still in the early stages of trials.
It may be difficult to hear, but, overall, it’s good that vaccines (and other therapeutics, in general) take a long time to develop. This makes sure that they actually work and that they don’t come with unwanted and severe side-effects.
No matter how promising or how ingenious a candidate may seem, it will have to clear all the safety, efficacy, and regulatory hurdles before it sees use in clinics. This could take several grueling months, at the least.
Even so, it’s clear to Dr Dalmacio that a vaccine is the best way out of this – but also, that other, more holistic lessons need to be learned, too.
“What we are experiencing now reiterates that ‘prevention is better than cure,’” she says. “Like for most diseases, keeping a healthy lifestyle also helps in battling the infection if there is exposure.”
“There is nothing wrong with having many facets in battling the infection. The world just has to ensure that whatever vaccine or drug or treatment strategy is recommended, it has to be backed up with robust and valid scientific studies,” she adds. “Now is one of those times when we don’t want to mess up with science.”