In late 2002, a then-undescribed virus crossed the species barrier in southern China and found a welcoming home in the lungs of humans. Over the next eight or so months, it spread, at first quietly across the region, and then explosively across the globe. The disease, what we have come to know as SARS or the severe acute respiratory syndrome, would infect more than 8,000 people and kill hundreds.
Now, nearly two decades later, another of its kin jumped that same chasm and has taken the world hostage.
Science is better now than it was then. Within days of detection, the genome sequence of the novel coronavirus (which eventually would be named SARS-CoV-2) was up online. Almost by the hour, solid scientific information about the virus is being published.
This has, in turn, powered large, cross-border collaborative efforts to cure and contain the disease, COVID-19.
But this may also prove to be too much, especially for almost everyone else who doesn’t have specialized, granular knowledge of infectious diseases. For the majority of people, even just a basic, fundamental understanding of how viruses work―and how they kill―should help in parsing through snake oil remedies and sensationalist headlines.
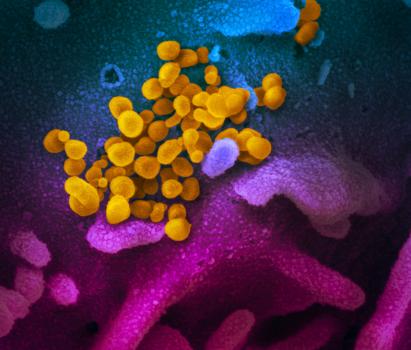
This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (blue/pink) cultured in the lab. Photo credit: NIAID-RML
Technically, viruses aren’t even alive. They are tiny infectious particles that, like parasites, rely on other, actually living cells to survive and reproduce. Their bodies usually just consist of a hollow protein shell, sometimes also coated with fat, housing their genetic material. Coronaviruses―a family of viruses to which the culprits behind SARS and COVID-19 belong―are pretty much the same.
On its surface, coronaviruses are studded with protrusions called spike proteins. Under the microscope, they resemble “the solar corona,” according to the virologists who first documented them in 1968. Inside, the SARS-CoV-2 genome is a single-stranded RNA molecule around 30,000 bases long, just like its cousin from the early 2000s. For perspective, the human genome has approximately three billion base pairs.
Very broadly speaking, the virus contains the code to create two classes of proteins. Proteins in the first form the shape of the virus. This group includes the spike proteins, which make up the virus’ shell along with the envelope and membrane proteins. The nucleocapsid proteins, on the other hand, protect the viral genome, keeping it safe and stable inside.
The second class comprises the non-structural proteins (NSPs, for short). As the name suggests, this group fulfills roles beyond giving the virus its overall form. Mostly, they see use inside the infected cell, where they hijack the host’s own machinery for the virus’ own use. Some NSPs also help the virus hide from roving guards of the immune system.
Even with a shallow arsenal, viruses are crafty and potent, often managing to stay ahead of their hosts in an evolutionary arms race.
Hitching a ride on tiny droplets, SARS-CoV-2 finds its way into the body through the mouth, the nose, or the eyes. As air blows through the throat, past the voice box, and down the windpipe, the virus latches on to and infects the cells it passes. In the days that follow, and through one way or another, the virus finds itself in the lungs.
Aside from giving SARS-CoV-2 its signature shape, the spike protein acts sort of like a key that allows the virus entry in to a cell. The lock that it opens is a protein called the angiotensin-converting enzyme 2 (ACE-2, for short). Among the many other roles it fulfils, ACE-2 is a transmembrane protein, which means it’s embedded in the cell membrane, so that one part of it is inside the cell while another is outside. This lets it relay external information internally―a crucial function that SARS-CoV-2 exploits.
As it turns out, the airway is full of cells that bear ACE-2. As the virus arrives, it’s not going to have much trouble finding a target to infect.
Inside an infected cell, the virus sheds its protein coat and frees its genetic material. Then, through the work of the different NSPs, the coronavirus slowly commandeers the host, uses its native resources and apparatus, and turns it almost into a factory. Simultaneously, the viral genome is copied many times over and the structural proteins are produced. These are then assembled into new virions that exit the host to go on and infect other cells.
“Millions of new viruses can be released from infected cells,” says Leslie Dalmacio, PhD, President of the Philippine Society of Biochemistry and Molecular Biology, and a professor at the UP College of Medicine. “So, one can imagine that if the virus dose upon infection is high, there will really be a high viral load in an infected person.”
At a certain point, likely when its value has run out, the virus orders the host to kill itself. The cell then dismantles its infrastructure, packs everything up in bags of cell membrane, and dissolves away. As the infection progresses, this process repeats itself over and over, sweeping across the lungs and killing thousands of cells along the way.
The body, of course, does not just sit idly and let the destruction happen unchecked. According to Denise Bascos, PhD, Associate Professor at the UP Diliman National Institute of Molecular Biology and Biotechnology, the fight begins almost immediately.
“[T]he immune response starts once the virus enters your nose, mouth, or eyes,” she says. In the earliest stages, the airway coats itself with mucus, making it tough for the virus to hook onto ACE-2. The body, in an attempt to mechanically expel the virus, also starts coughing and sneezing.
“This initial response is similar for all viruses and bacteria that enter through the oral and nasal cavities, which is why the symptoms are so generic,” Dr Bascos explains. In a lot of cases, though, this initial response isn’t enough.
To fight off infections, the body routinely resorts to a complex physiological safeguard called inflammation. As SARS-CoV-2 destroys scores of cells in the lungs, the cells cry out for help, sending out a stew of chemical distress signals, very broadly classified as cytokines.
Different cytokines will have different roles to play, but the overall effect is that a nearby blood vessel dilates and its walls become leaky. In turn, blood and other fluids stream into the area. This explains the redness, swelling, and heat in the moments after sustaining a wound.
“When the cytokines reach the brain, this may cause fevers that help control and fight off the infection,” Dr Bascos adds.
More importantly, the blood brings with it cells of the immune system, which then swarm the infected tissues, killing and devouring the invading pathogens. The ensuing war leaves a mess of dead cells and fluid in its wake, which clogs the lungs and makes breathing difficult.
In most patients, this is enough to clear the virus. Under proper supportive care, the inflammation recedes and the patient recovers shortly thereafter.
But in critical and extreme cases of COVID-19, “the immune system responds drastically with what we call a cytokine storm,” Dr Dalmacio says. “The cytokines keep on being released from cells of the immune system, and so inflammation ensues.”
How exactly SARS-CoV-2 throws the immune response into disarray is still mostly unknown, but, over time, the inflammation goes haywire and turns against the body, producing some very abrasive chemicals that further compromise the lungs and make it vulnerable to other, opportunistic infections seeking to cash in on the catastrophe.
At this point, the patient will have already fallen deeper into the disease, developing some of COVID-19’s most lethal and terminal symptoms―such as acute respiratory distress syndrome and respiratory failure―and will need invasive ventilation to stay alive. According to one early study, more than 90 percent never recover.
Editor’s Note: The situation continues to unfold at a rapid pace, and scientific findings cited in the article should be viewed accordingly.



