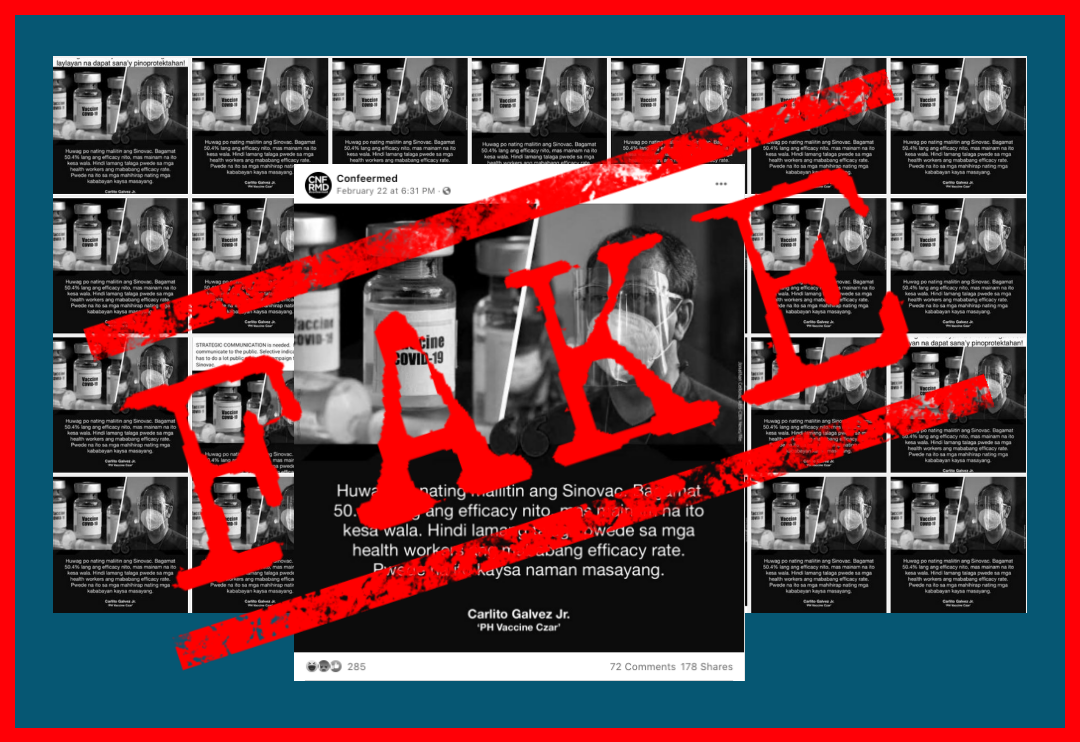
Several Facebook (FB) accounts have revived this month a fake list of eligibility requirements for inoculation with CoronaVac, the COVID-19 vaccine of Sinovac Biotech Ltd. It was attributed to “Beijing’s Vaccine Prevention Center” and an alleged specialist from Hong Kong, and enumerated 14 restrictions for vaccination with the China-made jab.
The supposed restrictions covered 1) those who have anemia, malignant tumors, mental health disorders, hereditary diseases, and “unstable” cardiovascular diseases; 2) those who have undergone and are recovering from major operations; 3) those who have suffered from a stroke; 4) those who are taking antiviral medicine and immunosuppressants, among others.
There is no truth to the list, which first circulated among Filipinos in March. Its source is a sham, and its contents do not align with guidance from health authorities and Sinovac.

The Department of Health (DOH) has also debunked the post and called it “fake” the first time it made the rounds online. They reshared this notice when the post began circulating again starting Aug. 13.
The DOH said CoronaVac may be administered to people with controlled comorbidities such as cardiovascular disease, cancer, malignancy, and diabetes. For people who have autoimmune disease, are transplant patients, or are on steroid treatment, among others, they are required to get medical clearance before vaccination.
Meanwhile, people who have received blood products like convalescent plasma may get CoronaVac after 90 days.
Source is a sham
There is no Beijing Vaccine Prevention Center. It does not appear in any cursory search for health agencies in the Chinese capital. The local health authorities in Beijing are its Center for Disease Prevention and Control and its Municipal Health Commission.
More guidance contradicts claim
The World Health Organization (WHO) gave the go-ahead for emergency use of CoronaVac in adults aged 18 and older on June 1. It said the vaccine is recommended for persons with comorbidities known to increase a person’s risk of getting severe COVID-19. These include obesity, cardiovascular disease and respiratory disease.
The only individuals listed by the WHO as ineligible for vaccination are:
- those with a history of having a serious allergic reaction to any component of the vaccine;
- those who had COVID-19 who have not yet recovered and have not yet met the criteria to end their isolation period; and
- those with a body temperature of over 38.5°C.
There is not enough data to assess the vaccine’s efficacy and possible vaccine-associated risks in pregnant women, said the WHO, but its effectiveness for pregnant women is “expected to be comparable to that observed in non-pregnant women of similar age.”
Sinovac’s product information, available on the Philippine Food and Drug Administration (FDA) website, carried the same three recommendations the WHO made about who may not get the vaccine.
The fake post re-circulated among Filipino Facebook users a day after two million more doses of SinoVac bought by the government arrived in Manila on Aug. 12.
The fabricated guidelines were posted by multiple pages and private accounts on Facebook. One of its most popular copies was published Aug. 15 and has gotten over 23,000 shares and 3,600 reactions. It has also been reposted by several province-based accounts, including the FB page Marinduque Transport Service Cooperative – Matrasco and the public FB group called Mandaue City.
(Editor’s Note: VERA Files has partnered with Facebook to fight the spread of disinformation. Find out more about this partnership and our methodology.)