(Photo Source: Twitter of World Health Organization – Western Pacific)
Three years after doing mostly administrative work as director general of the Food and Drug Administration (FDA), Dr. Rolando Enrique “Eric” Domingo found the job “boring” and “tiring.” Last December, he decided that it was time “to go in another direction in [his] career.”
On Jan. 3, the first working day for 2022, Domingo’s resignation took effect. “I’m relaxed, yes,” he said in an informal conversation with VERA Files via Zoom three weeks after he left the FDA.
He is back at the University of the Philippines College of Medicine, teaching ophthalmology, eye cancer, and pathology, and treating patients at the Philippine General Hospital (PGH).
“I believe I did my part to help during the pandemic. The FDA is now stronger, more efficient and systems are in place,” Domingo said when news about his resignation came out.
Domingo became a familiar face on television and online news outlets during the coronavirus disease 2019 (COVID-19) pandemic because the FDA was one of the busiest government offices, having the responsibility of approving all of the country’s COVID-related supplies – from face masks, test kits, alcohol, to drugs and vaccines – responding to it.
“It’s been three years, I mean, the medicines are available, the vaccines are available. It’s just a matter of implementing the programs now. At least, we have removed [the agency’s] notoriety for corruption, we’ve speeded up the process, and these are now in place,” he told VERA Files in a mix of Filipino and English.
“After a while, it’s a boring job; it’s very administrative. I wanted another kind of work,” he added.
The Philippines launched its vaccination program in March 2021 at the PGH, where Domingo was one among the first batch of healthcare workers who were inoculated.
To date, the FDA has approved nine vaccine brands for emergency use in the country. (See A show of trust: Philippine public health officials receive first jabs of Sinovac vaccine)
In the conversation with VERA Files, Domingo said, “I got some good results, and I got some not so good results, so on to the next na.”
One of the unfavorable results, he shared, is the languishing bid of the Department of Health (DOH), FDA, and other health advocates for tighter regulation of heated tobacco products (HTPs) and electronic nicotine device systems (ENDS), more known as e-cigarettes and vapes.
Although he had left the FDA where he carried the rank of a Cabinet undersecretary, Domingo said he will remain staunch in fighting for tobacco control, adding that, “Wala namang use ang tobacco na healthy in any way, so talagang natural na kalaban.”
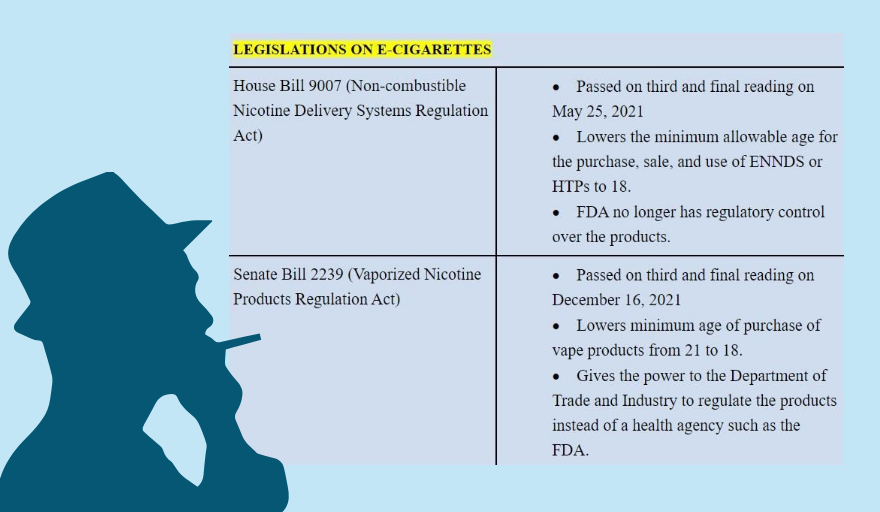
The Senate and the House of Representatives ratified on Jan. 26 the compromise version of Senate Bill (SB) No. 2239, approved on Dec. 16, and House Bill (HB) No. 9007, passed last May 25, that lowers the age of access to novel tobacco products from 21 years old to 18, strips the FDA of the regulatory authority over them, removes the two-flavor limit on electronic juices or liquids, among other provisions.
Under SB 2239, the authority to regulate vapes and e-cigarettes is given “exclusively” to the Department of Trade and Industry (DTI). In HB 9007, regulation is under the DTI in consultation with the FDA. The compromise version carried the House proposal.
Currently, devices for vaping and e-cigarettes fall under the DTI while the FDA regulates electronic juices and other products used in alternative smoking.
“The products have a pharmacological effect on the body, meaning the vapes, and the liquids. ‘Yun ang sinasabi naming hindi pwede sa DTI dahil meron ‘yang effects sa health ng tao,” Domingo explained.
If President Rodrigo Duterte signs the vaping bill into law, it will reverse the policies set in Republic Act (RA) No. 11467, specifically the provision that prohibits selling of HTPs and ENDS to persons aged 21 and below, and Executive Order No. 106 that mandates the regulation of these products to the FDA.
RA 11467, promulgated in January 2020, has a transitory period of 18 months.
If the new measure is vetoed, the FDA has already prepared a team dedicated to fully implement the 2020 law, specifically the provisions pertaining to registration and regulation, according to Domingo.
However, he said he does not expect that the process will be an easy one, noting: “Every time the DOH or FDA does anything to try to regulate such products, laging very strong ang resistance niyan.”
Tobacco companies and pro-tobacco groups have argued that vapes and e-cigarettes are “less harmful” alternatives to conventional smoking; hence, helpful in smoking cessation.
Scientists have noted that e-cigarettes are not without harm to human health. These were found to heighten exposure to toxic substances and carcinogens found in combustible tobacco cigarettes and increase risks of illnesses such as cardiovascular disease, cancer, and other adverse outcomes.
The exact health effects from long-term use of such products, especially among younger users, are not yet clear, according to the World Health Organization (WHO) Regional Office for Europe (Read VERA FILES FACT SHEET: What lowering age restrictions on e-cigarette smoking means for the youth).
“Talagang wala namang such a thing as ‘less harm’ e; harmful lahat yan, yung produkto na ‘’yan. Hindi naman ka magiging mas malusog kung gumamit ka ng HTP o ng vape,” Domingo said. “Ang malusog ‘yung hindi gumagamit.”
(There is really no such thing as ‘less harm;’ all of these products are harmful. You won’t be healthier if you use HTP or vape… The healthy ones don’t use these [products].)
The FDA, including Domingo, became a target of criticism during congressional hearings for its position against e-cigarettes and vapes. Some legislators accused the agency of corruption for allegedly accepting money from The Union, a known anti-tobacco nonprofit organization headquartered in Paris, France.
It was, in fact, a 2016 grant for a research project to develop an “evidence-based regulatory framework” for e-cigarettes and vapes.
The funds, which amount to $150,430, were allotted to “cover all operational expenses,” including hiring personnel to conduct the research, FDA Director for Center for Cosmetics Regulation and Research Anna Rivera explained during a June 9, 2021 congressional hearing.
“Ang DOH at saka ‘yung FDA will always work for public health and that also means working always against the use of tobacco,” Domingo said, admitting that the “relentless” attacks were one of the reasons that made him feel tired of the job at the agency.
“Well, it is tiring and thankless. I knew that even when I came in. But this time, I felt that I have given everything, I have done everything,” he said. “You just have to be steadfast and you just have to really know naman what your goals are.”
“Health systems all over the world keep feeling the pressure” from the tobacco industry, Domingo pointed out, in spite of the World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC).
One of the provisions of the convention, ratified by the Philippine Senate in 2005, states that: “In setting and implementing their public health policies with respect to tobacco control, Parties shall act to protect these policies from commercial and other vested interests of the tobacco industry in accordance with national law.”
The FCTC also restricts its parties from receiving any form of tobacco sponsorship in international events, activities, and their participants.
The bicameral conference committee on the vaping bill, however, removed the control on sponsorship.
“Sponsorships are now allowed even beyond industry associations and trade events… It also allows (tobacco) companies to conduct corporate social responsibility-related activities,” according to Sen. Pia Cayetano when she manifested her disagreement to the ratified version of the bill. She said this is “in clear violation” of the WHO FCTC, to which the Philippines is a signatory.
In November 2021, the Philippine delegation to the ninth Conference of the Parties (COP) to the WHO FCTC did not reach a consensus on issues such as the impact of tobacco on public health and the health harms of e-cigarettes. Still, Foreign Secretary Teodoro Locsin Jr. read the statement supportive of the industry at the five-day conference.
To this, the DOH and FDA expressed their strong disapproval of the position statement read by Locsin, who said that tobacco is a “source of good through taxation.”
(Read Philippine government promotes tobacco industry interests at global health forum; civil society outraged and VERA FILES FACT SHEET: The PH gov’t stance on tobacco industry, novel products)
Domingo, who was among the 10 co-heads of the delegation, refuted this and said: “Whatever money is made from the tobacco industry is not enough to cover the health costs of the people who are getting sick and dying from tobacco.”
According to the DOH, the Philippines is one of 15 countries worldwide “with a heavy burden of tobacco-related ill health.”
Every year, 87,600 Filipinos die from tobacco-related diseases, costing Php 188 billion from healthcare expenses and foregone income from getting sick and premature death due to lung cancer, chronic obstructive pulmonary disease, heart disease, and stroke.
“Ang gusto nga natin hindi siya i-normalize, it should not be acceptable to be vaping, to be exhaling these perfume smoke,” Domingo said. “It should not be something that children would have access to, kasi once na ma-addict na sa nicotine, tuloy-tuloy na ‘yan (once you get addicted to nicotine, it’s unceasing).”
The FDA has received the first case report of a vape-associated lung injury in November 2019, which involved a 16-year-old female who had been using e-cigarettes for six months and, at the same time, smoking combustible cigarettes.
Domingo said there have been a few reported cases of acute lung injuries because these are new products. “Siguro makikita pa natin ‘yan (We may be seeing that) 10 years from now,” he added.
While health groups lament the government’s position at the COP9, Domingo and other experts are still hopeful that the current bill will be dismissed. They have called on President Duterte to veto the bill in its entirety and allow the existing laws to prevail with tighter regulatory provisions on novel tobacco products.
(Read Vape bill goes against Duterte’s vow to fight addiction – medical practitioners)
Domingo assured VERA Files that he will continue to pursue anti-tobacco control advocacy, probably for at least another 25 years.
“Kailangan ka pa rin lumaban (You have to keep on fighting)… if you give up naman and you just let it go, parang kasalanan din naman (it’s like a sin)… kasi alam mo kung ano yung makabubuti at saka hindi (because you know what it good and bad),” he said.
When asked if he has any plan to become a congressman or senator, the resigned FDA chief said: “Wala po. Alam niyo talagang napakahirap na buhay n’yan; pagdodoktor ang alam ko (None. You know that’s really a difficult life; what I know is being a doctor).”
This story is produced under the project ‘Seeing through the Smoke’, which has support from the International Union Against Tuberculosis and Lung Disease, Inc (The Union) and Bloomberg Philanthropies.