(UPDATED) In declaring tobacco as a health product, the Supreme Court (SC) has made “far-reaching” strides in interpreting tobacco laws and regulation, according to a former health undersecretary.
“With this ruling saying that the Framework Convention on Tobacco Control (FCTC) is now actionable without need of any other legislation, it has become part of the law of the land in accordance with our Constitution,” lawyer Alexander Padilla said in a July 14 press briefing organized by HealthJustice.
The FCTC, under the guidance of the World Health Organization (WHO), is an evidence-based treaty that prescribes a regulatory strategy to address the tobacco epidemic. The Philippines ratified the WHO FCTC in September 2003 and the Senate gave its concurrence in June 2005.
Padilla, a Health undersecretary from 2003 to 2010, said tobacco “should be treated as a health product” under the regulation of the Food and Drug Administration (FDA) and the Department of Health (DOH), and not as a trade commodity under the Department of Trade and Industry (DTI).
On June 13, the SC released its 37-page ruling in favor of the FDA as the agency that has jurisdiction over the health aspects of tobacco products.
It reversed a 2012 decision by the Regional Trial Court of Las Piñas- Branch 255, which granted the petition of the Philippine Tobacco Institute (PTI) to prohibit the enforcement of the implementing rules and regulations of Republic Act (RA) 9711 or the FDA Act of 2009.
“This is a landmark decision of the Supreme court. Ang takeaway nga natin dito, paulit ulit nating sasabihin na (Our takeaway here is that, and we will keep repeating that) tobacco products are health products. And, as such, they are health products, they are regulated by the FDA and DOH… [The Supreme Court decision] also recognized the application of the [FCTC]. The FCTC is the one and only treaty engineered by the [WHO] and it only affects tobacco,” Padilla asserted.
With the Supreme Court ruling, Padilla said, DTI should “remove [tobacco] as part of any commodity and make it a health product.”
Benedict Nisperos, lawyer and legal consultant of HealthJustice, explained that the SC interpreted two basic laws: RA 9711 and RA 9211 or the Tobacco Regulation Act of 2003.
“If we’re going to a paradigm shift that health would be the priority – and it should be the Health Department and the FDA [that will] regulate these products – [RA] 9711 is sufficient,” he said.
But Nisperos added that for the FDA to get full authority over tobacco products – meaning, to include regulating even advertisements, sponsorships, and promotions – RA 9211 still needs to be amended.
The PTI has claimed that Section 25 of RA 9211 gave the Inter-Agency Committee Tobacco (IAC-T) the “exclusive jurisdiction over tobacco products, including its health aspect.” The DTI heads the IAC-T and the DOH is vice chair.
Senior Associate Justice Marvic Leonen underscored in his decision that PTI, which represents the major transnational tobacco companies in the country, is, in effect, proposing “an interpretation of our law that will effectively remove them” from the regulation of local health authorities.
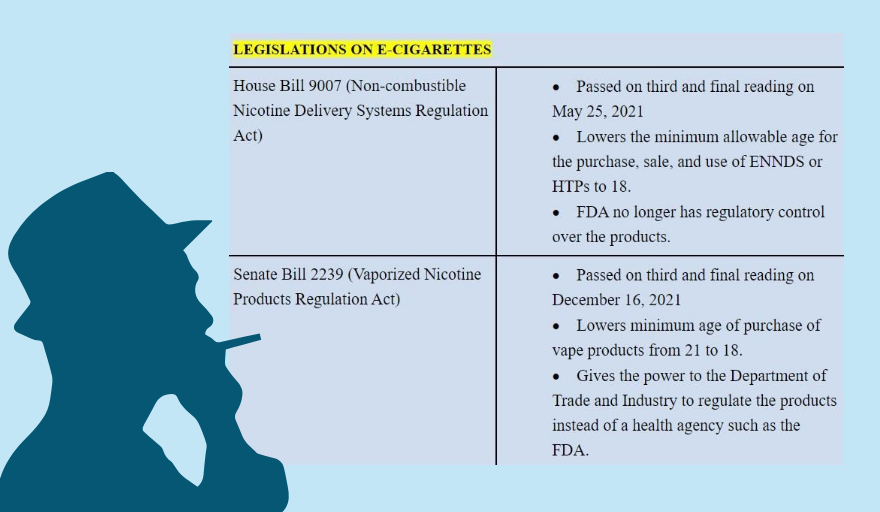
For this reason, health advocates are wary of the pending vape bill that could lapse into a law if President Ferdinand “Bongbong” Marcos Jr. does not veto it before July 24.
The bill, transmitted on June 24 to the Office of the President, weakens the stringent regulations of Republic Act (RA) 11467, a tax measure enacted in 2020 that assigns FDA as the sole regulatory authority over novel tobacco products, including e-cigarettes and heated tobacco products. (Read Vape bill version 2022: Congress ‘hijacks’ stringent regulations and Emasculating the FDA)
“It’s actually quite absurd that vape products and even tobacco products would be regulated by the Department of Trade and then all other tobacco products will be regulated by the FDA when e-cigarettes and heated tobacco products are also harmful to health,” said Dr. Ulysses Dorotheo, executive director of the Southeast Asia Tobacco Control Alliance (SEATCA).
According to Dorotheo, lowering the age restrictions on vapes and HTPs from 21 years old to 18 and allowing more than two flavors in the market, which are some of the controversial provisions of the vape bill, could attract new smokers and make it difficult for current users to quit.
(Read DOH, FDA win in SC could go to waste if vape bill becomes law)
Padilla echoed Dorotheo’s position and emphasized that the vape bill will only “defeat the very purpose also of the [SC] decision.”
Editor’s note: This article was updated to correct the name of the Philippine Tobacco Institute (PTI). We apologize for this mistake and we thank the reader who pointed it out.