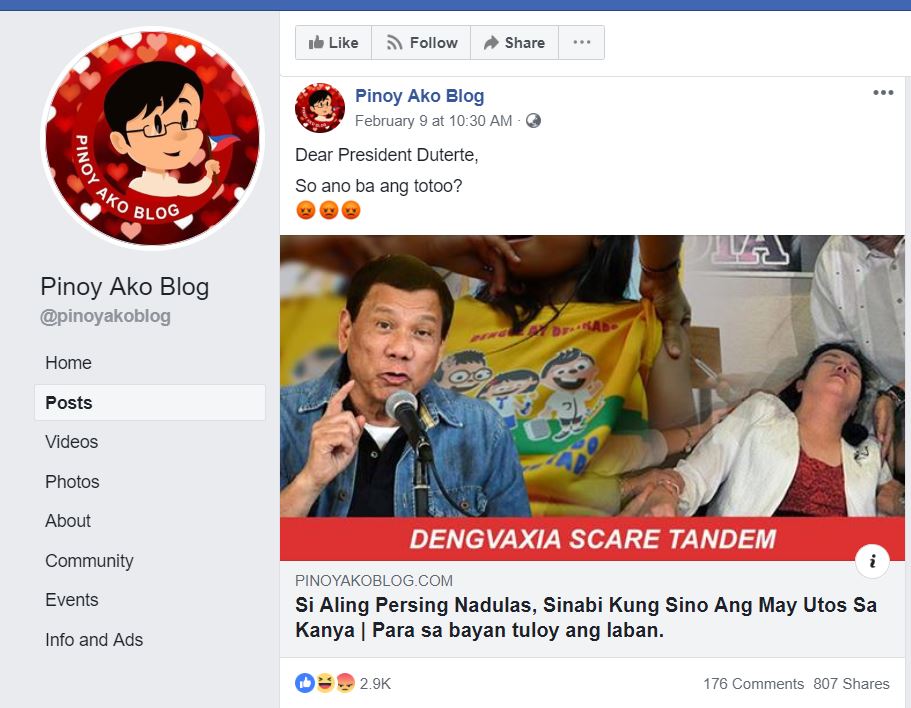
The chief of the forensic laboratory at the Public Attorney’s Office (PAO) has made inaccurate claims about a 2014 clinical study on the Dengvaxia vaccine as local health authorities call for a review of the evidence on inoculations against dengue fever.
The Philippines is facing a dengue surge, logging a total of 82,597 cases as of July 16 this year, according to the latest data from the Department of Health (DOH).
STATEMENT
In a July 14 interview with broadcaster Noli De Castro on ABS-CBN’s TeleRadyo, Dr. Erwin Erfe, PAO’s forensic laboratory division head, claimed that the proposition to revisit the studies on Dengvaxia is “dangerous.”
Citing a 2014 study on the clinical efficacy and safety of the vaccine, Erfe said:
“Clinical trial po ito. More than 10,000 pong mga kabataan ang sinaksakan dito. Napakababa po ng efficacy, 56% lang. At six out of 10 na sasaksakan mo ng Dengvaxia ay magsa-suffer ng severe adverse effects, anim out of 10.”
(This was a clinical trial. More than 10,000 children were vaccinated. The efficacy was very low, just 56%. Six out of 10 who were vaccinated with Dengvaxia will suffer severe adverse effects, six out of 10.)
Source: TeleRadyo, PAO binatikos ang panawagang gamitin ulit ang Dengvaxia sa kabila ng rising dengue cases, watch from 5:06 – 5:28
Then, he added:
“Kasama po dito ‘yung deaths. ‘Yun nga po ‘yung apat na nakamamatay na sinabi ng Sanofi, dinisclose po sa FDA (Food and Drug Administration) na itinago naman po ng Department of Health sa mga doktor at nabakunahan. Hindi po alam ito. Lumabas lang po ito nung Senate hearing, ‘yung disclosure na ‘yun na meron palang four severe adverse effects ang Dengvaxia: ‘yung multi-organ failure, neurotropism, at ‘yung severe dengue, at saka ‘yung anaphylactic reaction.”
(This includes deaths. There are four (effects of Dengvaxia) that can prove fatal, according to Sanofi, that it disclosed to the FDA but which the Department of Health hid from doctors and vaccine recipients. This was not known. It was only revealed during the Senate hearing, this disclosure that there are four severe adverse effects from Dengvaxia: multi-organ failure, neurotropism, severe dengue, and anaphylactic reaction.)
Source: watch from 5:28 – 6:03
As of Aug. 3, the video of Erfe’s interview has gained 4,786 views on YouTube.
FACT
Erfe referred to a randomized controlled trial, published in the peer-reviewed journal The Lancet on July 10, 2014, that looked into the level of protection against symptomatic dengue after getting a Dengvaxia vaccine 28 days after the third dose. The study, conducted between June 3 and Dec. 1, 2011, involved 10,275 healthy children aged 2 to 14 years old from Indonesia, Malaysia, Thailand, the Philippines, and Vietnam.
Sanofi Pasteur, manufacturer of the Dengvaxia vaccine, sponsored the study.
Health experts from Meedan, a global technology nonprofit, emphasized that the research was “not the final study used to guide recommendations” on Dengvaxia. To date, Dengvaxia is recommended for persons aged 9 to 45 years old who have once been infected with the dengue virus.
VERA Files Fact Check flagged three of Erfe’s statements that are false, misleading, and needs context, respectively:
| CLAIM | RATING |
| The Dengvaxia vaccine, developed by Sanofi, can cause four severe adverse effects: multi-organ failure, neurotropism, severe dengue, and anaphylactic reaction. | FALSE |
The study does not specifically document any cases of these so-called adverse effects, according to Meedan.
“In fact, the study directly states that no cases of allergic reactions or neurotropic disease were reported,” the health experts explained, adding that “dengue was much less likely to be severe among individuals who were vaccinated that had breakthrough infections.”
The most common non-serious side effects from the vaccine include soreness, itchiness or pain on the injection site, headaches, lack of energy, and general discomfort.
| CLAIM | RATING |
| Six out of 10 of those who get Dengvaxia vaccine suffer severe adverse effects. | MISLEADING |
The researchers recorded 647 serious adverse events, including 402 or 5.99% of 6,710 participants who got the Dengvaxia vaccine and 245 or 7.31% of those who got the placebo.
“Serious adverse events were consistent with medical disorders in this age group and were mainly infections and injuries,” the researchers said.
In an email to VERA Files Fact Check, Meedan explained, “As we can see from breaking down these data, the percentage of people who experienced serious adverse events was actually greater in the control group and the numbers are not even close to what the claim is stating.”
| CLAIM | RATING |
| The clinical trial, which involved 10,025 children aged 2 to 14 years old, indicated a “very low” 56% efficacy rate for the vaccine. | NEEDS CONTEXT |
As of July 14, the World Health Organization (WHO) requires vaccines to have a “high efficacy rate of 50% or above.” To be approved as a vaccine, both high efficacy and high safety are needed.
The WHO noted that Dengvaxia has an efficacy rate of about 80% among children within the age range of 9 to 16 years old with previous dengue infection. This means that 8 out of 10 of those in this age bracket who get the vaccine “will likely not get infected with dengue after getting vaccinated.”
However, the global agency said in a Dec. 22, 2017 article that “the vaccine was not licensed in children younger than 9 years because of the less favorable safety and efficacy results in young children.”
Meedan’s health experts wrote in a July 21 explainer that “Dengvaxia was found to be effective against all four dengue virus serotypes,” in particular, 89% against DEN-4 and 67% against DEN-1 and DEN-2.
BACKSTORY
In 2019, the Food and Drug Administration permanently revoked Sanofi Pasteur’s certificate of product registration for Dengvaxia because of its failure to submit complete post-approval commitment documents, including risk management plans (RMP).
The DOH said in a statement on Aug. 22, 2019 that “the efficacy of Dengvaxia itself is not an issue in this case.”
“Given that Dengvaxia is an innovative drug, the importance of complying with these post-marketing commitments is critical to public safety,” said former Health secretary Francisco Duque III.
“The DOH found that while Sanofi has submitted the first and second versions of the RMP, it has failed to submit the third version and has belatedly submitted the fourth version, in violation of its post-marketing commitments and pertinent FDA rules and regulations,” he added.
Have you seen any dubious claims, photos, memes, or online posts that you want us to verify? Fill out this reader request form.
Sources
Department of Health, DOH Beat COVID-19 Media Forum, Aug. 2, 2022
On DOH’s plans to review Dengvaxia vaccine
- CNN Philippines, Health expert calls for reconsideration of Dengvaxia use amid rising dengue cases, July 11, 2022
- Inquirer.net, Gov’t should review Dengvaxia vaccine amid rising dengue cases — expert, July 11, 2022
- GMA News Online, Salvana urges open mind on Dengvaxia amid rise in dengue cases, July 14, 2022
On the surge of dengue cases
- Malaya Business Insight, 83% hike in dengue cases this year, July 20, 2022
- Inquirer.net, Dengue cases over 65,000; 274 deaths, July 19, 2022
- Business Mirror, DOH logs 83% jump in dengue cases from January 1 to July 2, July 19, 2022
The Lancet, Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial, July 10, 2014
U.S. National Library of Medicine, Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial, July 10, 2014
World Health Organization, Vaccine efficacy, effectiveness and protection, July 14, 2021
World Health Organization, Updated Questions and Answers related to the dengue vaccine Dengvaxia® and its use, Dec. 22, 2017
Meedan Health Desk, What do we know about the safety of Dengavaxia vaccine, July 21, 2022
Meedan Health Desk, Personal communication, July 28, 2022
On the revocation of Dengvaxia’s certificate of product registration
- BusinessWorld Online, FDA permanently bans Dengvaxia, Feb. 19, 2019
- ABS-CBN News, FDA permanently revokes Dengvaxia registration, Feb. 19, 2019
- Inquirer.net, FDA permanently revokes Dengvaxia’s certificate of registration, Feb. 19, 2019
Department of Health, DOH UPHOLDS FDA, DENIES SANOFI APPEAL ON DENGVAXIA CPRs, Aug. 22, 2019
(Guided by the code of principles of the International Fact-Checking Network at Poynter, VERA Files tracks the false claims, flip-flops, misleading statements of public officials and figures, and debunks them with factual evidence. Find out more about this initiative and our methodology.)